In a world increasingly concerned about reducing carbon emissions, the shift towards electricity as a greener power source is well underway. However, this transition extends beyond just cars and into the vast global manufacturing network that produces a wide range of products, from batteries to fertilizers. To make this shift sustainable, researchers from the University of Chicago have made a groundbreaking discovery in the field of electrochemistry, finding a way to use electricity to enhance chemical reactions commonly used in synthesizing pharmaceutical drugs. Their findings, published in Nature Catalysis, not only advance the field of electrochemistry but also pave the way for designing and controlling reactions more efficiently, ultimately making the chemical industry more sustainable.
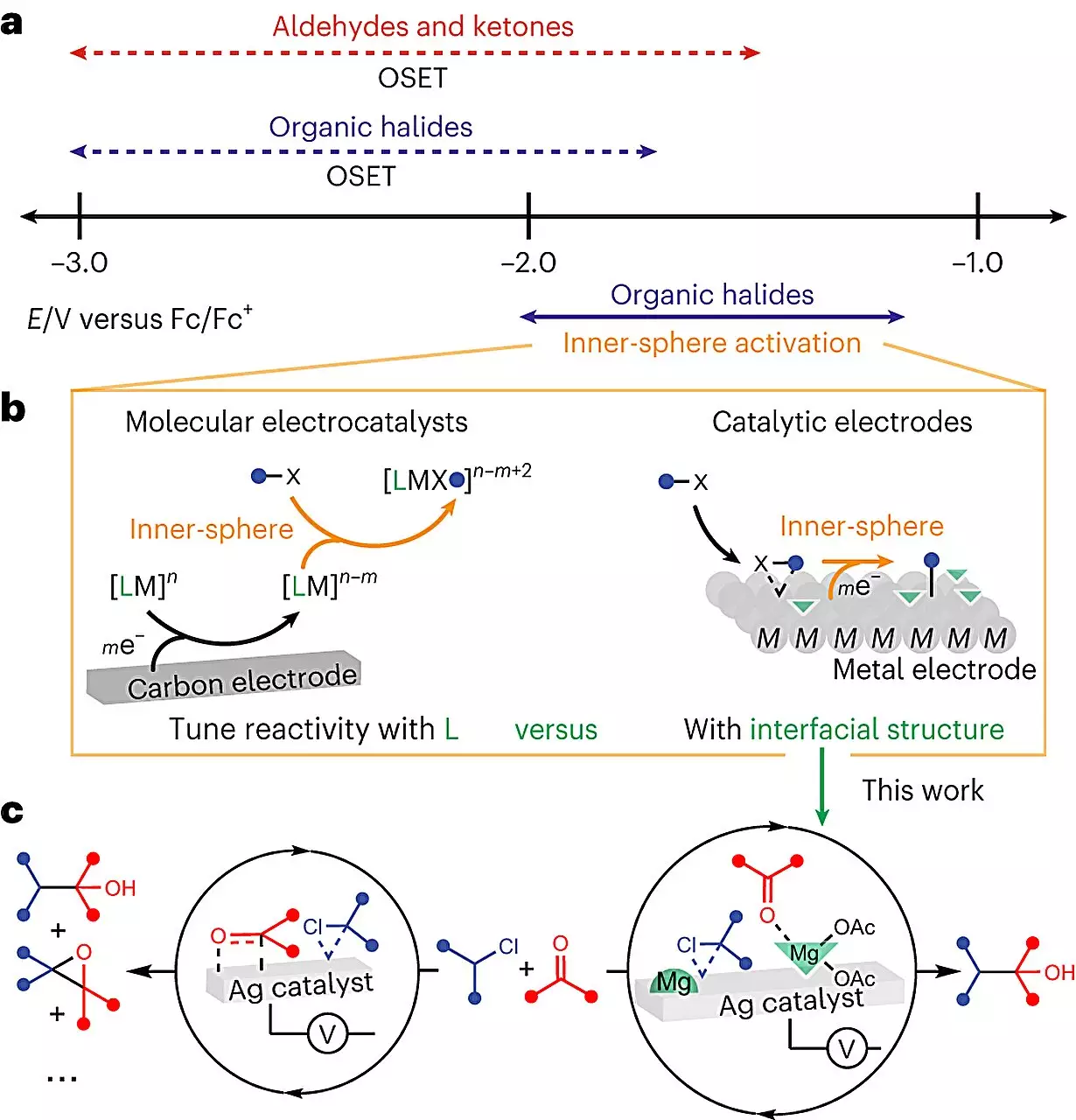
Electrochemistry, as a scientific field, is notably complex due to the intricate molecular interactions involved. Inserting a conductive solid, known as an electrode, into the reaction mixture to provide electricity adds another layer of complexity. Scientists have been grappling with understanding the roles each molecule plays and the order in which they interact, making an already intricate process even more convoluted. This is where Anna Wuttig, the Neubauer Family Assistant Professor at the University of Chicago, sees an opportunity.
Professor Wuttig proposes viewing electrochemistry as a unique design lever that unlocks possibilities not found in other systems. By focusing on the electrode’s surface, which is responsible for providing the electrical input, Wuttig and her team aimed to unravel the catalytic role it plays. They zoomed in on a specific type of reaction frequently used in manufacturing medicinal chemicals, aiming to create a bond between two carbon atoms. While theoretical predictions suggested a 100% yield when performing this reaction with electricity, lab experiments often yielded lower results.
The team hypothesized that the presence of the electrode tempted some molecules away from their intended destinations during the reaction. To counteract this, they introduced a Lewis acid, a chemical compound, into the liquid solution. The Lewis acid redirected the molecules to the desired reaction sites, resulting in a near-clean reaction. The researchers also employed sophisticated imaging techniques to visualize and understand the molecular-level mechanisms. This breakthrough allowed them to demystify the complex interfacial structure and gain insights into the reaction process instead of regarding it as a black box.
According to Professor Wuttig, this discovery is a crucial step towards not only utilizing electrodes in chemistry but also predicting and controlling their effects. It offers the potential for sustainable synthesis, with the added benefit of reusing the electrode for multiple reactions. In most reactions, the catalyst is dissolved in the liquid and is removed during the purification process. However, with the electrode, it can now be retained for subsequent reactions, further reducing waste and increasing efficiency.
As the world strives to reduce its carbon footprint, the pharmaceutical and chemical industries play a crucial role. By harnessing the power of electricity and unlocking the potential of electrochemistry, researchers are paving the way for a greener chemical industry. This breakthrough in sustainable synthesis not only offers a more efficient and controlled approach to chemical reactions but also contributes to the overall sustainability of the manufacturing process.
The University of Chicago’s research on the use of electricity to boost chemical reactions is a significant breakthrough in the field of electrochemistry. By focusing on the fundamental interactions at the electrode interface, scientists are gaining valuable insights into designing more efficient reactions. This discovery sets the stage for a greener chemical industry, where renewable energy sources power electrochemical processes. As researchers continue to unravel the complexities of electrochemistry, the future holds the promise of sustainable synthesis and environmentally friendly manufacturing practices.

Leave a Reply