Water disinfection is a critical process in ensuring public health and safety. Traditional methods involving chlorine have been effective but come with their own set of challenges. Researchers are now looking towards scalable electrochemical ozone production (EOP) technologies as a more sustainable alternative. By generating ozone directly in water, EOP offers similar disinfecting power to chlorine but with the added benefit of natural decomposition after 20 minutes, reducing the risk of harmful exposure.
A key aspect of making EOP a viable solution lies in understanding the role of catalysts in the process. The research conducted by a team from the University of Pittsburgh and Drexel University delves into the molecular level interactions that occur during EOP. By investigating the interplay between catalyst corrosion and homogeneous reactive oxygen species, the researchers aim to uncover the mechanisms behind efficient, economical, and sustainable EOP technologies.
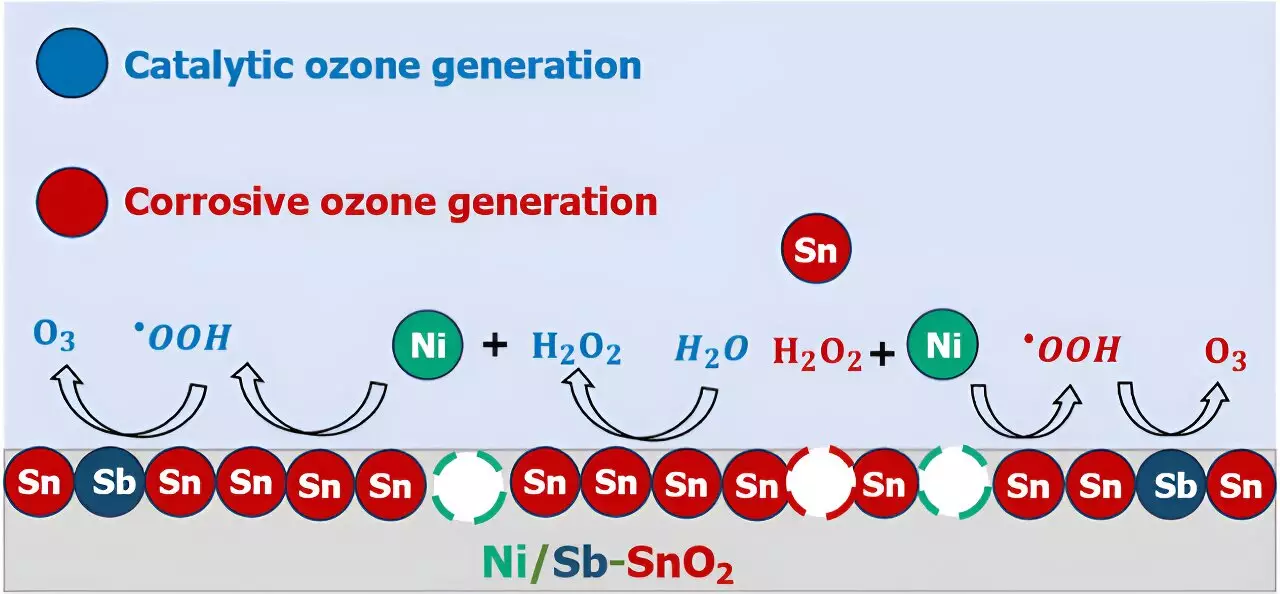
The researchers focused their attention on a promising EOP catalyst known as nickel- and antimony-doped tin oxide (Ni/Sb—SnO2 or NATO). Through a series of experimental and computational analyses, they were able to piece together an atomic-scale storyline of how ozone is generated on NATO electrocatalysts. Surprisingly, they found that the catalyst’s effectiveness is influenced by a combination of factors, including metal ion leaching, corrosion, and solution phase reactions, highlighting the complexity of EOP processes.
The findings of this study have significant implications for the future of water disinfection technologies. By identifying the key mechanisms at play in EOP catalysts, researchers can now pursue improvements in catalyst design to enhance the efficiency and sustainability of the process. It is crucial to address fundamental technological constraints such as corrosion and solution phase reactions before EOP can be implemented on a larger scale.
The potential of electrochemical water treatment on a global scale hinges upon the discovery of advanced catalysts that are both resistant to corrosion and conducive to economically viable EOP. By elucidating the intricate workings of EOP catalysts, researchers are paving the way for more sustainable and scalable water disinfection solutions that can benefit both modern cities and remote villages alike.
The quest for sustainable water disinfection through electrochemical ozone production represents a crucial frontier in environmental engineering. By decoding the molecular mysteries of EOP catalysts, researchers are taking a step towards revolutionizing water treatment processes worldwide. It is imperative that further research and innovation continue in this field to realize the full potential of EOP technologies in ensuring clean and safe water for all.

Leave a Reply